Here
at Ask Erik, we've spent a lot of time reading books and comics,
watching movies, and browsing through the Internet in the hopes of
finding the answers to life's biggest mysteries. What are the odds of a meteor strike on Earth? Why can't food scientists ever agree on what's healthy and what isn't healthy? Why hasn't The Philanthropist been put on DVD yet?
Having instead amassed a vault of useless knowledge stored in his head, Erik instead tackles your questions and tries to find the answers you care about (or a reasonable facsimile). Or, if you don't care, he'll at least try to make you laugh and forget you just wasted time you could spend doing anything else.
So, last time I took a serious topic and actually treated it with respect, answering a question about how mankind can look for planets that can support life, or even look for advanced forms of life itself.
This week, I was fully planning on answering a question that would have lead to a discussion about just how abominable the Muppet Babies were, but that will have to wait for another week because I received a follow-up question!
To Erik: From an evolutionary standpoint, why wouldn't life on other planets evolve without the use of oxygen?
For anybody who read my last article, you might remember that I indicated a major way to determine if there is life on other planets is to look for oxygen in the atmosphere, because oxygen only exists in a pure form on worlds that have life (plants taking carbon dioxide, emitting oxygen for those of you who remember biology 101).
Of course, why couldn't life evolve to breathe in other gases? After all, our air is mostly nitrogen (about 80% in most places around the planet), followed by Oxygen (about 20%), then at less than 1% each are argon, water vapor, carbon dioxide, neon, methane, helium, krypton, hydrogen, xenon, ozone, nitrogen dioxide, iodine, carbon monoxide, and ammonia.
Now, a few of those we can eliminate right off the bat. Ammonia and carbon monoxide are a bit too complex for a lot of organic creatures to breathe. Water vapor still contains oxygen, but it's still a bit too close to being a liquid to be reliable. As for the rest? Well, we're going to have to get small.
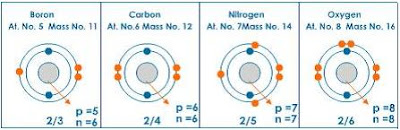
Everybody remember this guy? Don't panic, I'm not going to turn into your elementary school science teacher (you guys did learn about this originally in elementary school, right?). I just want you to take a close look at one particular section of the table. Let's see if we can zoom in on one section.
No, wait, come back! Seriously! This will be quick!
The three elements we're going to focus on here are carbon, nitrogen, and oxygen. Now, I'm not going to go into a lot of detail work here, but I will simply point out that oxygen is a high energy element and that nitrogen is a low energy element. What this means is that oxygen reacts much, much quicker with other elements than pretty much any other element that exists (look at all the chemicals that end in "ite" or "ide." That means there's oxygen there).
So what does this mean for us? Well, when we breathe in a lungful of gas, we're taking in that whole mixture, which then needs to bond with carbohydrates to create energy for us to move our bodies. Since oxygen bonds much, much faster than other gases, it makes sense that, from an evolutionary standpoint, we'd become creatures that go for the easy way out.
Plus, I'll point out that we have discovered creatures that do feed off of other elements. Beans have microorganisms inside them that feed off nitrogen. There are creatures at the bottom of the ocean who feed off the gases that come from volcanic vents. What they have in common is that they aren't very big, because it's so much harder to extract energy from other gases and use it to fuel their bodies. Does this mean a creature couldn't be man-sized and breathe some other gas? ...well, I suppose not, but as we understand biology right now, I'm not sure how that would adapt to get as much energy as we require.
So, why did I include carbon in this mess? Well, to quote Jon Stewart, "I'm a huge carbon guy. I enjoy the molecular slut of the table of elements. It will bond with anything. It's ridiculous. And by the way, four bonds."
Most life on this planet is carbon-based. Like oxygen, it bonds with other chemicals at a ridiculous level. When a plant "breathes" it's taking in carbon dioxide and water (and you actually need water for life to form in, last I heard), and converting it into sugars and oxygen. Those same sugars that fuel plants also fuel humans, but we take ours in by eating other things instead of just taking it from the air because we can't perform photosynthesis.
But, again, those sugars and carbs both contain carbon and oxygen, which are key in providing energy to us.
But there's one other thing I need to point out. Having 20% oxygen in the air is about all we can handle. Breathing in too much oxygen can be just as toxic to people as breathing in pure neon gas at certain levels, so it's important to keep that in mind.
So, how does this relate to my last question? Well, last time I mentioned that oxygen only exists in its natural state in a world that has life. Is it plausible some other world exists where creatures roam around breathing iodine, maybe. But if we see oxygen in the atmosphere, we know that life exists there, because we only reached 20% oxygen in our atmosphere after billions of years of converting water and carbon dioxide to oxygen.
It is possible we'll miss another planet with life on it if we don't see oxygen there, but considering how expansive space is, we'll probably want to focus our attention on more sure bets once we have the technology to closely examine planets and (hopefully) one day travel to them.
...man, this is serious stuff. I can guarantee you that tomorrow's article will most certainly not be anywhere near this level of sophistication.

No comments:
Post a Comment